Abstract
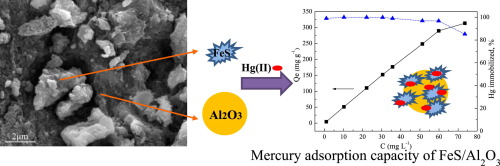
Iron sulfide (FeS) nanoparticles were known for their excellent ability to scavenge aqueous Hg(II). However, the aggregation and oxidizability of FeS nanoparticles greatly restricted their practical application. This study developed an Al2O3-supported nanoscale FeS (FeS/Al2O3) which effectively optimized the material performance. Characteristic data suggested that FeS/Al2O3 (mass ratio of FeS to FeS/Al2O3 is 30%) with a large specific surface area (142.7 m2 g−1) could evenly distribute FeS, suppressing the aggregation of FeS successfully. The maximum enrichment capacity of Hg(II) on FeS/Al2O3 achieved ∼313 mg/g at pH 6 and 30 °C. The pH studies indicated that alkaline environment strongly inhibited mercury uptake, while removal rate of 1 mg/L Hg(II) maintained a high level (>97.5%) over the pH range of 3–9. However, FeS/Al2O3 loss was observed at low pH due to the acid corrosion. Additionally, the rates of Hg(II) reduction were almost unaffected over the range of 0.1–100 mM NaCl, but greatly inhibited by the presence of humid acid (HA). The synthetic FeS/Al2O3 remained high mercury removal efficiency (over 95% in 1 mg/L Hg(II)) and low iron dissolution rate when preserved for 30 days, showing good long-term stability and remarkable application potential.
Graphical abstract